Are all homogeneous mixtures also solutions?
1 Answer
All solutions are homogeneous mixtures, but not all homogeneous mixtures are solutions.
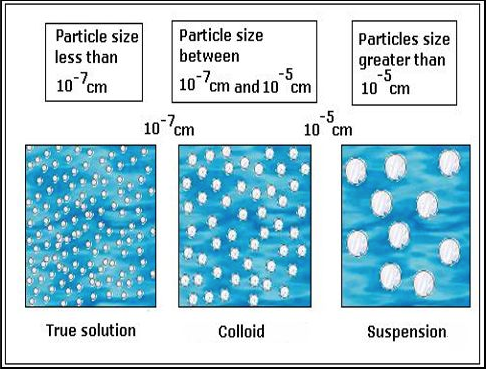
When a homogeneous mixture contains only one phase, then it is a solution. That is then the product of completely dissolving a solute into a solvent, without any undissolved particles. That makes it one phase, because no solid is tangible or visible.
An example of a homogeneous mixture that is not a solution is homogenized milk. It is uniform throughout for practical intents and purposes, but it is not a solution because there is a solid and a liquid phase in it, and you can see the "solid".
The "solid" is suspended in solution in the form of colloids (colloids are very, very tiny particulates, on the order of

