Question #5b5f7
2 Answers
See explanation.
Explanation:
When comparing the ionization energies of two elements we should consider four main factors:
- Distance from nucleus.
- Effective nuclear charge.
- Shielding effect.
- Electron-electron repulsion.
Consider the electron configuration for:
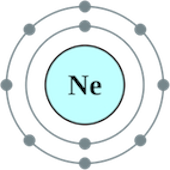
When looking at the electron configurations of
- Distance from nucleus: The electron removed from sodium is being removed from a higher energy level
n=3 than that of Neonn=2 . - Effective nuclear charge : Even though sodium's nuclear charge is
+11 and that of Neon is+10 , however, due to the distance from the nucleus, the nuclear charge effect is very minimal in this case. - Shielding effect: The electron removed from sodium is shielded from the attraction of the nucleus by
10" electrons" however, the electron removed from Neon is shielded by2" electrons" only. - Electron-electron repulsion: The electron repulsion effect is usually considered when the electrons removed are at the same distance from the nucleus and they are subjected to a similar nuclear charge and shielding effect. In this case, the electron-electron repulsion effect is minimal.
Here's how you can think about this.
Explanation:
As you know, ionization energy is the energy needed to remove a mole electrons from a mole of atoms in their gaseous state.
"X" + color(blue)("energy") -> "X"^(+) + e^(-)
This process produces cations, or positive ions.
What is important to note here is that the first ionization energy of an atom tells you how much energy is needed to remove its most loosely held electron.
So, what factors could influence the first ionization energy?
Well, the further away this outermost electron is from the nucleus, the easier it will be remove it, so right from the start atoms that have larger atomic radii will have lower ionization energies.
Moreover, as you move down a group in the periodic table, the outermost electrons are screened more efficiently from the nucleus by the inner electrons.
In your case, sodium's outermost electron is located on the third energy level, in the 3s-orbital.
By comparison, neon's outermost electrons are located on the second energy level, in the 2p-orbitals.
This means that neon is a smaller atom than sodium, so its outermost electrons are held more tighly by the nucleus
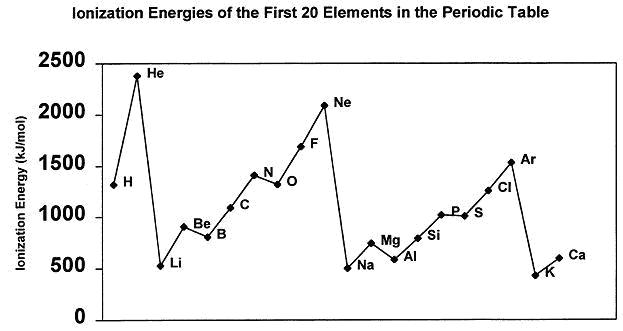 http://hrsbstaff.ednet.ns.ca/dawsonrj/11%20Chem/112%20Chem/Chapter%20Notes/Chapter%207%20Periodic%20table.htm
http://hrsbstaff.ednet.ns.ca/dawsonrj/11%20Chem/112%20Chem/Chapter%20Notes/Chapter%207%20Periodic%20table.htm
Not only does sodium have its outermost electron further away from the nucleus than neon does its outermost electrons, but it's screened better as well.
Therefore, the first ionization energy of neon will be higher than that of sodium.


