Question #5c428
1 Answer
Explanation:
You know that isotopes are atoms that have the same atomic number, which is given by the number of protons in the nucleus, but different mass numbers, which are given by the number of protons and of neutrons in the nucleus.
SImply put, for a given element, an isotope can be distinguished using the mass number alone, since by definition the number of protons is always the same.
In your case, iodine-126 refers to an isotope of iodine that has a mass number equal to
If you take a look at a periodic table, you will notice that iodine has an atomic number equal to
In isotope notation, the atomic number of the isotope is written in the bottom left of the atomic symbol. The mass number is written in the top left of the chemical symbol.
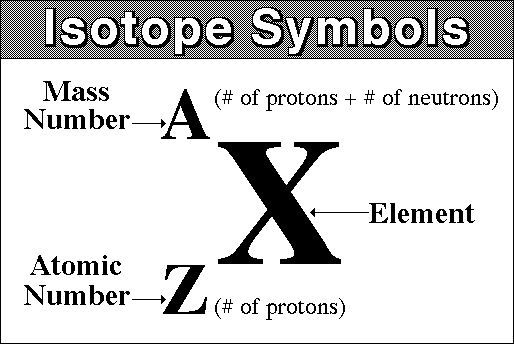
So, what would that look like in iodine-126's case?
Well, if its atomic number is
#""_(color(white)(x)53)^126"I" -># iodine-126
So keep in mind, when you ar egiven an isotope, you are automatically given that isotope's mass number, so look for its atomic number in the periodic table, then use this notation to write out the isotope.

