When might it be better to report percent changes in mass, instead of absolute changes in mass?
1 Answer
Then again, it might be less revealing sometimes to speak in
For example, maybe you want to report mass loss from thermogravimetric heating. When you do this type of analysis, you generally have milligrams of product on a tiny little scale and you get this curve of mass loss vs. temperature.
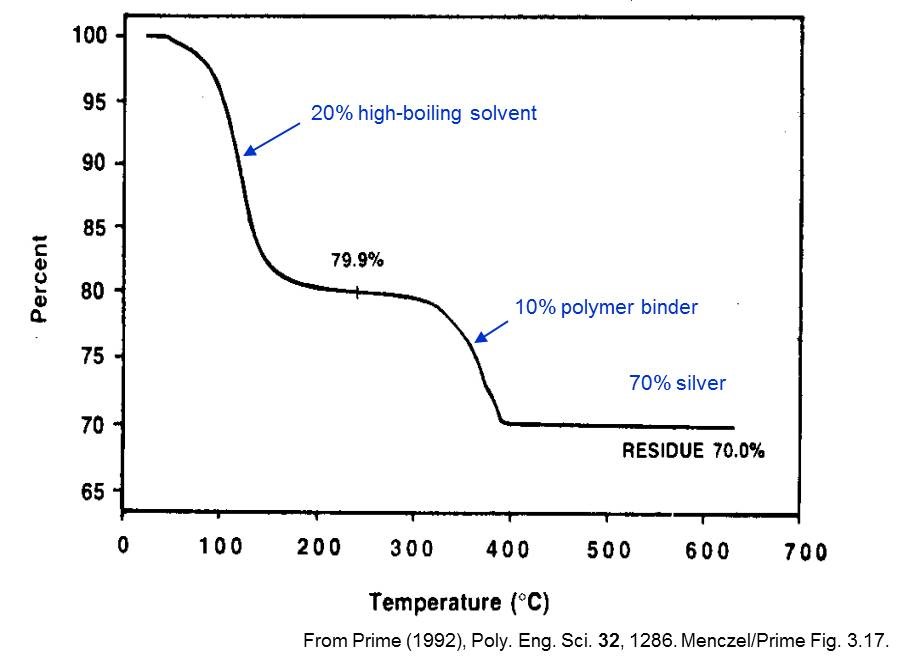
It is more useful to report this as a percentage because the quantities are so small, and because the ratio of mass lost on a small scale should be about the same ratio as mass lost on a larger scale anyway.
The intention here is to determine molecular formulas, which are scalable. Therefore, in this case, the percent is a way to report the data in a more accessible manner without the need to specify the scale.
Now, what if you had something on a large scale?
Maybe you have to report the amount of intermediate product lost during a synthesis process?
If you report the percent without the scale, you don't really know the significance of the loss of product. Furthermore, knowing the scale could help one rationalize how much larger of a scale would be practical, in terms of cost.
If you've lost a lot of product on a large scale, it would likely be a significant expense, and more importantly, it could detract from the viability of the synthesis process being efficient.

