What is the structure of #"C"_2"H"_3"O"_3#?
1 Answer
One structure is this terrible but hypothetically-possible three-membered ring called oxalic anhydride:

You can clearly see the amount of ring strain is horrendous on this molecule; the bonds have to unnaturally bend in order to form this structure, so you sometimes see these bonds being called "banana bonds". There is massive electron density on the carbonyls that promote repulsive forces on the ring. The resonance "stabilization" only creates further unfavorable repulsive forces that destabilize the unstable bonds even more.
This molecule has not been observed as of 2009, and is said to exist only hypothetically. I highly doubt under normal conditions that this molecule can be observed.
Another potential structure is this peroxoate, assuming that you are implying with or without a charge:
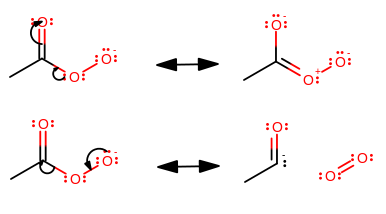
The resonance structures just aren't stabilized.
In the top resonance structures, the second oxygen is given a partial positive charge, which is unfavorable for an electronegative atom with
The bottom resonance structures are even worse. You never want to break apart the molecule, but that's what electron conjugation from the far-right oxygen would do---the

