Why does the carbonyl oxygen get protonated instead of the ether oxygen in the acid-catalyzed hydrolysis of amyl acetate?
1 Answer
Hydrolysis of an ester must start with the protonation of the carbonyl when under acidic conditions.
It is the carbonyl oxygen that has the most electron density here and thus for this mechanism acts as a good proton acceptor.
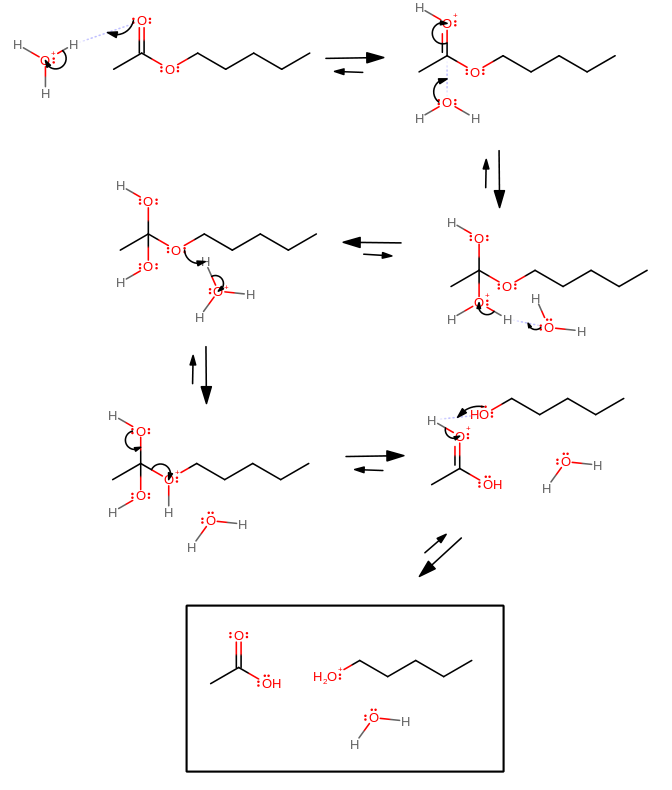
1. Protonate the carbonyl with protonated water.
2. Water can attack the electrophilic carbonyl carbon.
3. Water must transfer the proton to the proper leaving group, because that helps further the goal of this process.
4. Continue step 3.
5. The tetrahedral intermediate can then force the pentanol group to leave because it's now a good-enough leaving group.
6. The pentanol group, with a slightly higher pKa than that of water (
(Water may be protonated as well, but if given equal amounts of water and pentanol, pentanol would be protonated slightly more often.)

