What is ATP and how does it act as an energy-transfer agent? What is phosphoryl transfer potential?
1 Answer
ATP stands for adenosine triphosphate, which has three phosphate groups connected in a chain. The total charge of ATP is
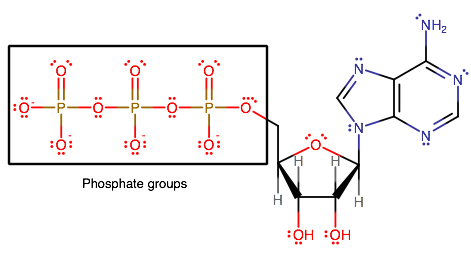
ATP therefore has phosphate groups it can transfer off of it. Phosphate,

After ATP transfers one phosphate group off of it, it becomes ADP, which stands for adenosine diphosphate, reflecting that it now has two phosphate groups connected in a chain.
The transferred phosphate carries with it energy stored within its bonds.
The greater the tendency or "potency" to transfer a phosphate, the greater the phosphoryl transfer potential is said to be.
The phosphoryl transfer potential of ATP is about

