How many moles of oxygen gas are required for the combustion of one mole of 3-methylpent-2-ene?
1 Answer
Explanation:
The first thing to do here is determine the chemical formula of 3-methylpent-2-ene.
You know that you're dealing with an alkene, hence the suffix -ene.
Moreover, you know that the parent chain is pentane, and that the double bond is placed on the second carbon. This means that you will have a methyl group,
The compound will look like this - I'll show you (E)-3-methylpent-2-ene, which is the (E) geometric isomer of 3-methylpent-2-ene
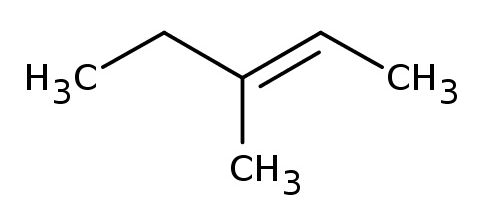
Notice that the compound's condensed formula can be written as
This means that you can represent the compound as
You're dealing with a hydrocarbon, which means that the combustion reaction will only produce carbon dioxide,
So, the balanced chemical equation for the combustion of 3-methylpent-2-ene looks like this
#"C"_6"H"_12 + color(red)(9)"O"_2 -> 6"CO"_2 + 6"H"_2"O"#
Notice that you have a
Therefore, one mole of 3-methylpent-2-ene will require nine moles of oxygen in order to react completely.

