Question #63258
1 Answer
Nov 20, 2015
The Lewis structure looks like this:
Explanation:
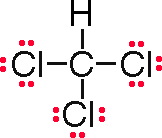
Each carbon atom has 4 unpaired electrons available for bonding.
Each of the 3 chlorine atoms has a single unpaired electron available for bonding.
The hydrogen has a single electron available for bonding.
This results in carbon forming 4 single covalent bonds.
The systematic name is trichloromethane.

