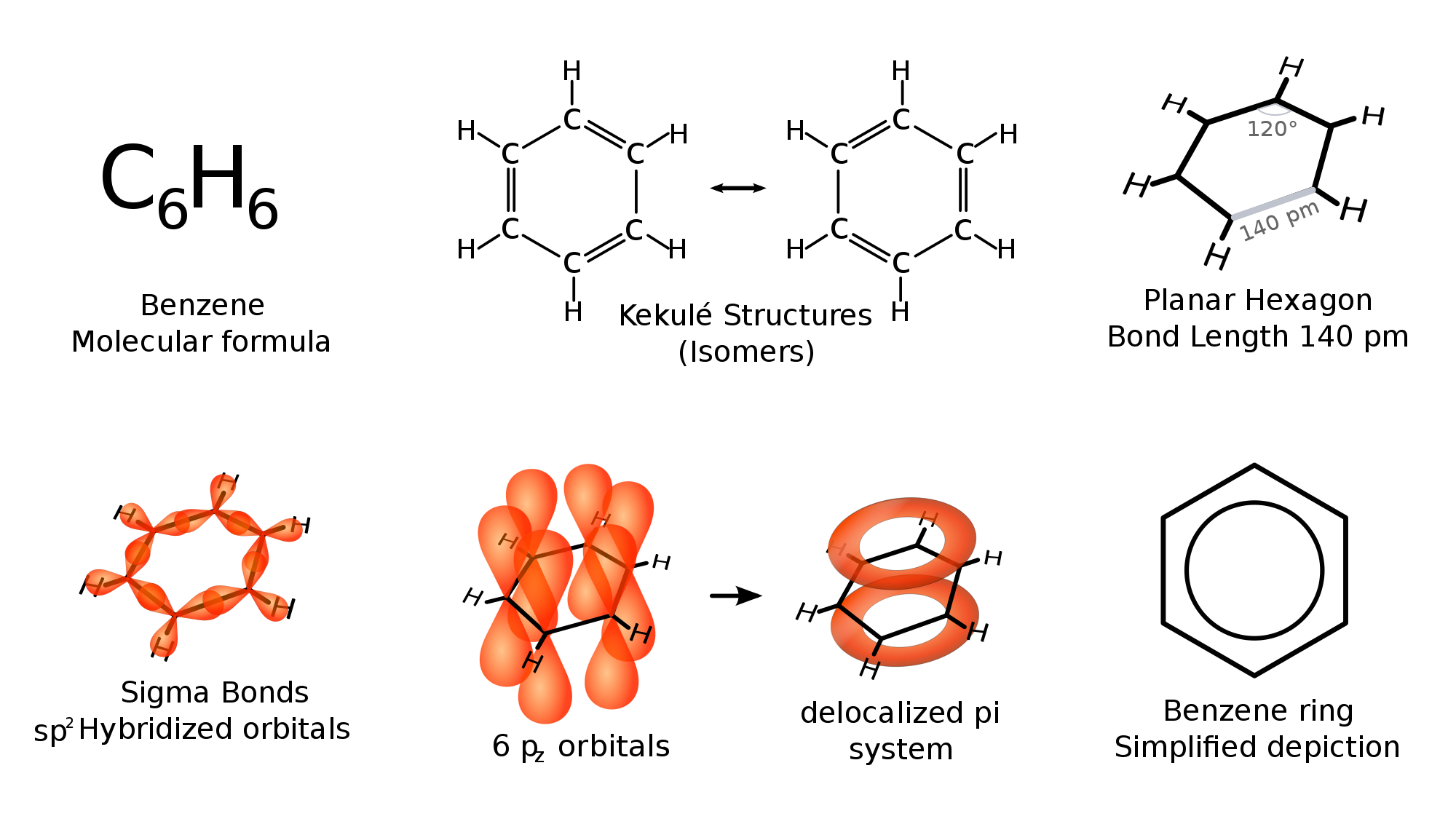Question #24d0b
1 Answer
Oct 26, 2015
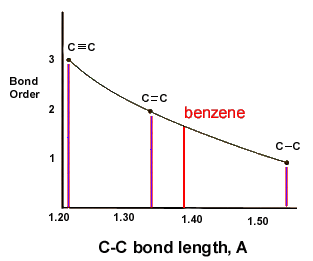
benzene does not have any double bonds
Explanation:
benzene or C6H6 does not have any double bonds. but instead it has bonds which falls between double and single bonds
The scientist Kekule proposed that benzene has two resonance structures which has three double bonds each. but later, according to the molecular orbital theory and hybridization, it was discovered that the real structure of benzene is almost similar to the resonance hybrid of the 'Kekule' structures.
take a look at the following images which explains the bonding of C atoms in benzene