How do we know what atomic number an element has???
1 Answer
If you know what the element is called... look it up.
You can always get the atomic number, as long as you know what element you're looking at. Always.
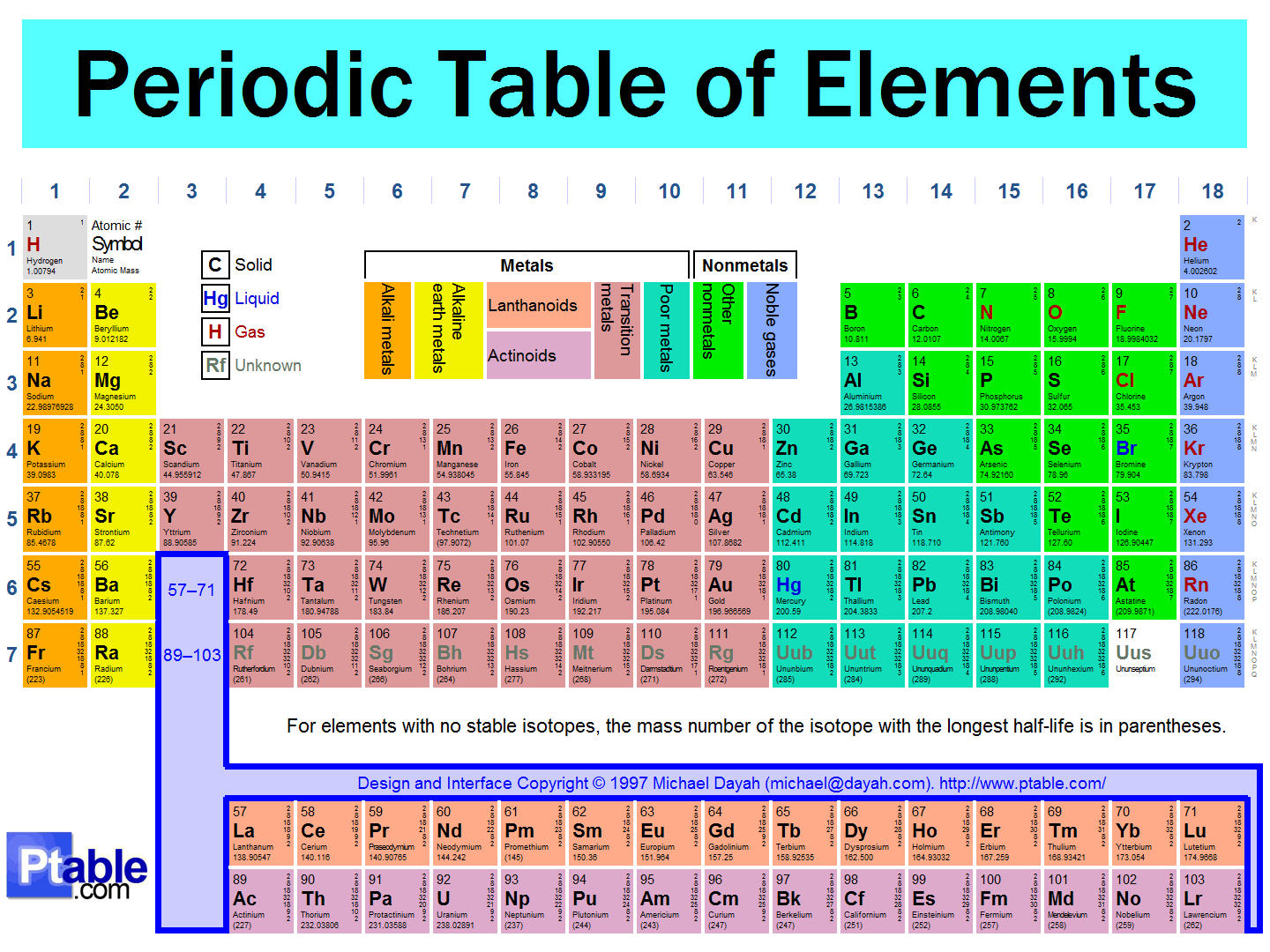 http://www.ptable.com/
http://www.ptable.com/
You can see the atomic number directly on each square. The relevant information from each square is:
"Z"
"X"
"Name"
"Atomic Mass"
For example, Phosphorus (
If the element is generally known to be stable, the most common isotope is the most stable.
So, we have:
"number of protons + number of neutrons = mass number"
"atomic number = number of protons"
"mass number - atomic number = number of neutrons"
31 - 15 = "16 neutrons"
Finally, the number of electrons has to be equal to the number of protons for an element in its elemental form, i.e. its natural, neutral state. So it must have

