Question #6735f
1 Answer
Explanation:
You can find the number of electrons in any neutral atom by taking a look in the periodic table.
More specifically, you're looking for the element's atomic number, which tells you how many protons said element has in its nucleus.
So how is that related to the number of electrons?
Well, a neutral atom will have equal numbers of protons, which are positively charged particles located in the nucleus, and electrons, which are negatively charged particles that surround the nucleus.
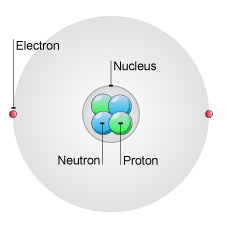
So, look for scandium,
This means that a neutral scandium atom has

