Question #60418
1 Answer
Explanation:
Based on the metal activity series, zinc is not active enough to replace the
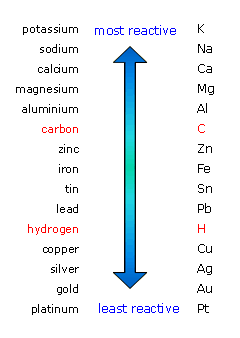 http://www.bbc.co.uk/schools/gcsebitesize/science/add_aqa_pre_2011/ions/electrolysisrev2.shtml
http://www.bbc.co.uk/schools/gcsebitesize/science/add_aqa_pre_2011/ions/electrolysisrev2.shtml
But laboratory results tells us otherwise because zinc (along with aluminum, copper, tin, lead, and beryllium) is considered as amphoteric, a molecule which reacts to bases and acids alike.
Also, the reaction is not an acid-base, as one might expect but more of an oxidation-reduction reaction with the following half-equations:
Oxidation:
Reduction:

