Alkyl halides react with excess ammonia to give what?
1 Answer
They should give some sort of tetraalkylammonium cation eventually. You might also call this a quaternary ammonium salt.
Since the pKa of a protonated alkylamine is generally higher than the pKa of ammonium (
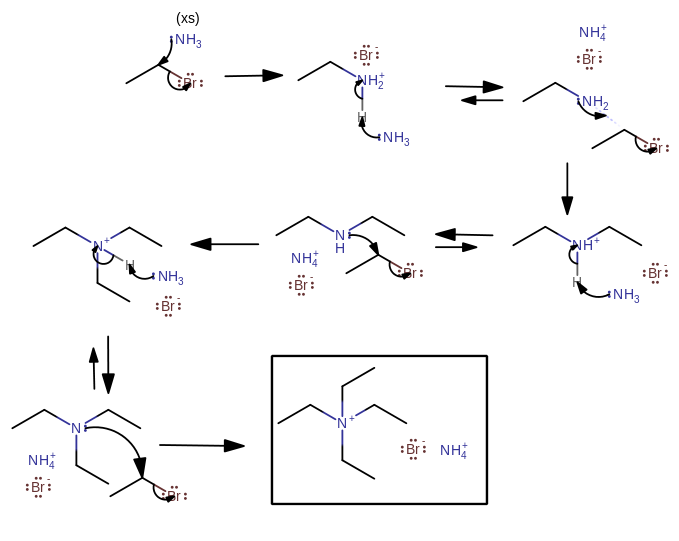
-
Initially the ammonia acts as the nucleophile.
-
Then, the created alkylammonium salt acts as the acid while the ammonia acts as a base (not a nucleophile!) that reactivates the alkylamine so that it can be a nucleophile.
- The alkyl halide remains as the electrophile and continues to attach until there are no more electrons for the alkylamine to donate. This is driven by the excess ammonia.
If you ever wondered what the point of this compound is... it's good as a phase transfer catalyst for dissociation of a compound and its subsequent transfer into a new type of solution. For example, it can transfer an inorganic compound from the aqueous phase into the organic phase.

