Question #ce86c
1 Answer
Think of the ethane molecule as two carbon-atoms surrounded by hydrogens, being replaced one by one.
Explanation:
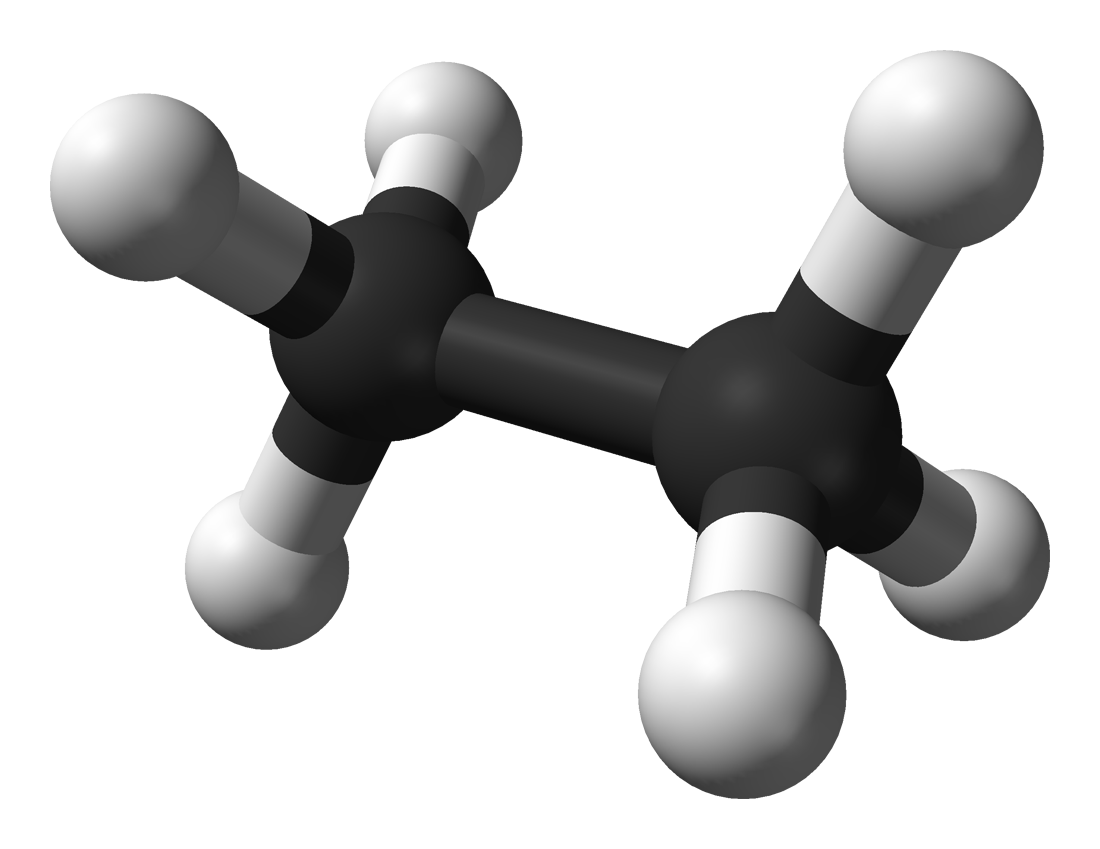
For the first
For the second you can choose to use the
For the third : if you start with the 1,1 version you can add on that
In short, if there is a majority on one of the
You should be able to work it out from here.

