Question #c8280
1 Answer
That's the concentration and temperature of the sulfuric acid solution.
Explanation:
You're dealing with a method used to prepare hydrogen peroxide,
Sodium peroxide,
The idea here is that once this reaction takes place, you can extract the sodium sulfate from the solution by cooling it about
The idea is that precipitating this hydrate will take away water from the solution in the form of water of crystallization for the solid.
This will in turn increase the concentration of hydrogen peroxide in the resulting solution. Think of it like this
#"same quantity of H"_2"O"_2 + "less water" -> "more concentrated solution"#
The sulfuric acid solution is used at ice cold temperature because hydrogen peroxide is notoriously unstable in aqueous solution.
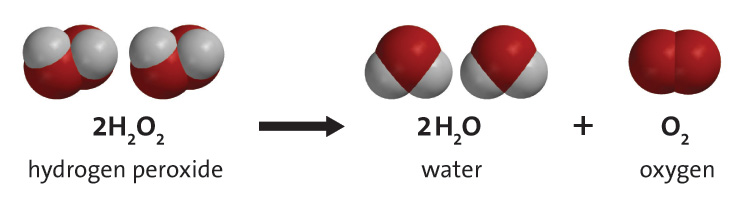
Its decomposition to oxygen gas and water is influenced by the temperature of the solution and by its concentration (the presence of a catalyst and impurities also influence this rate of decomposition).
You can use this method to get quite concentrated hydrogen peroxide solutions, so keeping the temperature very close to the precipitation point of the sodium sulfate decahydrate is preferable.

