Which of the following has the smallest bond order for #"CO"#? #["Mn"("CO")_6]^(+)#, #"Cr"("CO")_6#, #["Ti"("CO")_6]^(2-)#, #["V"("CO")_6]^(-)#
1 Answer
The
The more
CARBONYL LIGANDS ARE BOTH SIGMA DONORS AND PI ACCEPTORS
One of the most easily forgotten concepts here to remember is that
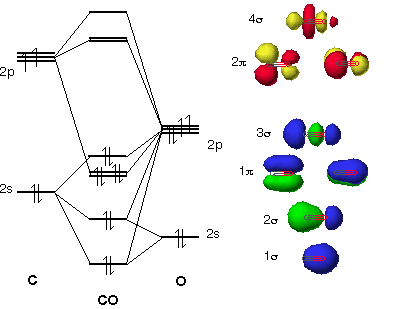
- Its
#sigma# HOMO is the one contributed to by the#2s# of carbon and the#2p_z# of oxygen (that's why there's only one HOMO, not two or three). - Its two
#pi^"*"# LUMOs are the second-highest-energy ones with contribution from the#2p_x# and#2p_y# of carbon and of oxygen.
METAL CHARGE, AND COMPLEX FORMED
1) Each
This is really saying that we have a
This is an octahedral complex because there are six ligands. In fact, all four of these complexes are octahedral.
2) Same situation, but with
3) Same situation, but with
4) Same situation, but with
Now, since we are talking about bond order, which correlates with the effective degree of the overall bonding (such as triple bonding being a bond order of 3, or
CHECKING TRANSITION METAL CHARGE AND RADIUS
Something definitely to consider here is the charge and radius of the metal. Here are the metals in order of ascending positive charge, and also decreasing radius:
#"Ti"^(2-) > "V"^(-) > "Cr"^(0) > "Mn"^(+)#
Now, what exactly would this do? At a glance, I would note that titanium is the largest and most negatively charged. That would imply that the internuclear distance is longer. Generally that implies a weaker
However, the largest anionic charge makes the metal the most electron-rich, and the most able to
Therefore, the

