In the production of methamphetamine from reducing epinephrine with "HI" in the presence of red phosphorus, what is the role of "HI", and could "HCl" be used instead? What is an accepted mechanism?
1 Answer
I had to do a bit of research on this (finding this and this), but as it turns out, iodine acts as a nucleophile in this circumstance.
We could suppose that the
One potential problem is that chloride is a worse leaving group than iodide because the pKa of
That implies that it would be a great idea to somehow have a driving force to get more hydroiodic acid to drive the equilibrium forward.
Red phosphorus acts as basically a catalyst; it reacts with the
I did find a schematic of what's probably going on, except it's with an alcohol:
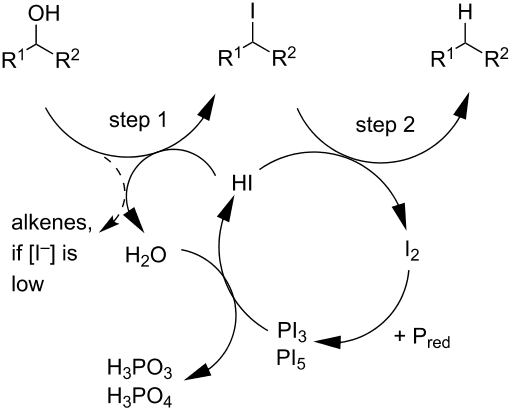 http://chemistry.stackexchange.com/
http://chemistry.stackexchange.com/
In the end, what you have essentially happening for OUR reaction is:
- The iodide acting as a nucleophile (mixture of
"S"_N1 and"S"_N2 ), except with no initial protonation necessary. - At some point,
"R"cdot and"I"cdot form. An"I"^(-) reacts radically with"R"cdot to form"R"^(-) and"I"cdot - The
"R"^(-) gets protonated. The two radical iodides probably reform"I"_2 , as"I"_2 is depicted in the above diagram to be "leaving" (produced in) the reaction to react with more red phosphorus.
One could draw out the final two aforementioned steps like so:
Ultimately you have reduced the alkyl halide into an alkane.
I personally have never heard of this reaction before (it's fairly new; 1982), and apparently it's quite dangerous (as are many radical reactions, such as peroxide radicals...), so maybe that's why I haven't been taught this reaction.
I wouldn't worry too much about the mechanism. In my opinion, no good professor in their right mind would put this on an exam. :)

