Question #93c80
1 Answer
Here's what I got.
Explanation:
Your strategy here will be to
- figure out what chemical element you're dealing with
- determine what
#57# and#139# actually represent
So, pull up a periodic table and look for the element with the chemical symbol

Now, notice that one of the two numbers given to you,
This number represents the element's atomic number, which tells you how many protons must be located in an atom's nucleus in order for that atom to be an atom of lanthanum.
So, any atom that has
Now for the second number,
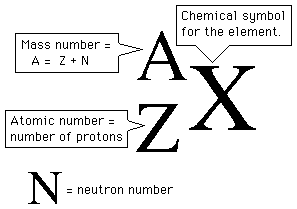
In your case, the lanthanum isotope given to you is written like this
#""_(color(white)(1)57)^139"La"#
This tells you that its atomic number is equal to
An isotope's mass number tells you how many protons and neutrons it contains in its nucleus.
#color(blue)("mass number" = "no. of protons" + "no. of neutrons")#
Since you know that you have
#"no. of neutrons" = 139 - 57 = 82#
Finally, the number of electrons found in a neutral atom will always be equal to the number of protons found in that atom's nucleus.
That happens because each electron carries a
In your case, the number of electrons found in a neutral lanthanum-139 atom is equal to

