Why do we use disodium EDTA?
1 Answer
Because generally, we don't need all six teeth to bind to a given target in practice, and because EDTA by itself is poorly soluble in water. Disodium EDTA is water-soluble.
EDTA, or ethylenediamminetetraaceticacid, a hexadentate, chelating ligand, looks like this:
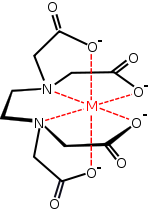
So you can see that four "teeth" on EDTA belong to the acetate oxygens, and two of them belong to tertiary amine nitrogens. EDTA has a high complexation formation constant, which means the equilibrium is highly in favor of EDTA binding.
However, EDTA by itself is NOT very water-soluble.
In disodium EDTA, or
This makes
In practice, it is generally more useful and convenient to have EDTA partially ionized as disodium EDTA since it is easier to dissolve in water for a complexometric experiment.

