How do we represent the #"sulfate ion"#?
1 Answer
Mar 5, 2016
Explanation:
The elements composing the
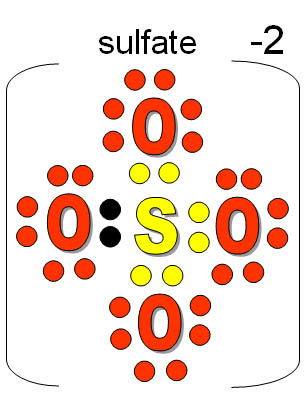
Sulfur shares its 6e- to 3 Oxygen atoms to complete the octet leaving 1 Oxygen with only 6. The oxidation number of
