Question #7fada
1 Answer
See explanation.
Explanation:
In order to be able to draw the Lewis structure for a given molecule, you need to know how many valence electrons it contains.
The total number of valence electrons available for a molecule is determine by taking into account the valance electrons each atom that is part of that molecule brings to the table.
A hydrogen sulfide molecule,
- one from each of the two hydrogen atoms
- six from the sulfur atom
The sulfur atom will act as a central atom, since it can form more than one bond. The hydrogen atoms will bond to the central atom via single bonds.
These bonds will account for
This will ensure that the central atom has a *complete octet.
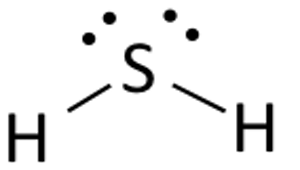
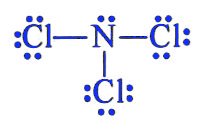
The exact same approach is used for nitrogen trichloride,
This means that you get a total of
- five from the nitrogen atom
- seven from each of the three chlorine atoms
Nitrogen will act as central atom here. The three chlorine atoms will bond to the central atom via single bonds. These bonds will account for
Each chlorine atom will complete its octet by adding
The remaining