#color(red)("Warning! Long answer!"#
All the credit for this answer goes to Stefan V.
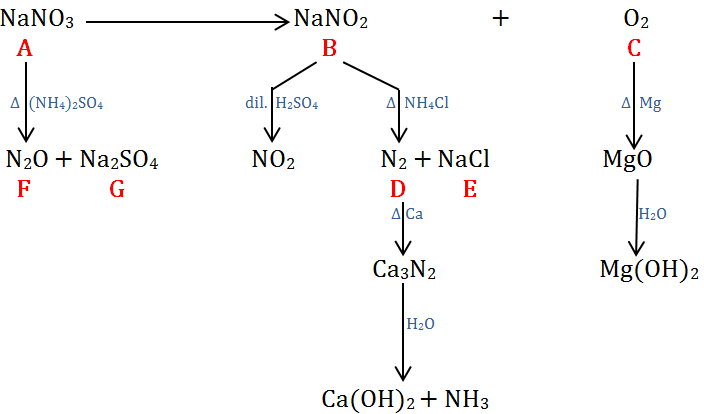
The big clues are the yellow flame colour (#"Na"#) for #bb"G"# and the brown gas obtained from #bb"B"# (#"NO"_2# or, less likely, #"Br"_2#).
#"NO"_2# comes from the decomposition of #"HNO"_2#.
This suggests that #bb"B"# is #"NaNO"_2# and that #bb"A"# is probably #"NaNO"_3#.
Acting on that assumption, we get
#2underbrace("NaNO"_3)_color(red)("A") stackrelcolor(blue)(Δcolor(white)(X)) (→) 2underbrace("NaNO"_2)_color(red)("B") + underbrace("O"_2)_color(red)("C")#
Then gas #color(red)(bb"C"color(white)(l) "is"color(white)(l) "O"_2)#.
#"O"_2# is a colourless gas that forms #"MgO"# when heated with #"Mg"#.
#"O"_2 + "2Mg" stackrelcolor(blue)(Δcolor(white)(m))(→) underbrace("2MgO")_color(red)("white powder")#
#"MgO"# is a basic oxide.
#"MgO + H"_2"O" ⇌ "Mg"^(2+) + "2OH"^(-)#
#color(red)(bb"B" = "NaNO"_2)#
#"NaNO"_2# reacts with sulfuric acid to form #"HNO"_2#, which decomposes to form the brown gas #"NO"_2#.
#2underbrace("NaNO"_2)_color(red)("B") + "H"_2"SO"_4 → "2HNO"_2 + "Na"_2"SO"_4#
#"2HNO"_2 → underbrace("NO"_2)_color(red)("brown gas") + "NO" + "H"_2"O"#
#color(red)(bb"D" = "N"_2)# and #color(red)(bb"E" color(white)(l) = color(white)(l)"NaCl")#
#"NaNO"_2# reacts with #"NH"_4"Cl"# to form #"N"_2# and #"NaCl"#.
#underbrace("NaNO"_2)_color(red)("B") + "NH"_4"Cl" → underbrace("N"_2)_color(red)("D") + underbrace("NaCl")_color(red)("E") + "2H"_2"O"#
#"N"_2# reacts with #"Ca"# to form #"Ca"_3"N"_2#.
#"3Ca" + "N"_2 stackrelcolor(blue)(Δcolor(white)(X))(→) "Ca"_3"N"_2#
#"Ca"_3"N"_2# reacts with water to form ammonia and #"Ca"("OH")_2#.
#"Ca"_3"N"_2 + 6"H"_2"O" → 3underbrace("Ca"("OH")_2)_color(red)("basic") + 2underbrace("NH"_3)_color(red)("colourless")#
#color(red)(bb"F" = "N"_2"O")# and #color(red)(bb"G" = "Na"_2"SO"_4")#
#"NaNO"_3# reacts with #("NH"_4)_2"SO"_4# to form #"N"_2"O"# and #"Na"_2"SO"_4#
#2underbrace("NaNO"_3)_color(red)("A") + ("NH"_4)_2"SO"_4 stackrelcolor(blue)(Δcolor(white)(X))(→) 2underbrace("N"_2"O")_color(red)("F") + underbrace("Na"_2"SO"_4)_color(red)("G") + "4H"_2"O"#
The #"Na"# in #"Na"_2"SO"_4# gives the yellow flame colour.
Formula Units of #bb"A"#
You don't give a pressure or temperature for the gas #bb"D"#, so I will arbitrarily assume that #P = "1 atm"# and #T = "25 °C"#.
Then, #n =(PV)/(RT) = (1 color(red)(cancel(color(black)("atm"))) ×0.120 color(red)(cancel(color(black)("L"))))/("0.082 06" color(red)(cancel(color(black)("L·atm·K"^"-1")))"mol"^"-1" ×298.15 color(red)(cancel(color(black)("K")))) = 4.90 × 10^"-3" color(white)(l)"mol"#
#"Moles of NaNO"_3 = 4.90 × 10^"-3"color(red)(cancel(color(black)("mol N"_2))) × (1 color(red)(cancel(color(black)("mol NaNO"_2))))/(1 color(red)(cancel(color(black)("mol N"_2)))) × ("2 mol NaNO"_3)/(2 color(red)(cancel(color(black)("mol NaNO")))_2) = 4.90 × 10^"-3" "mol NaNO"_3#
#"Formula units NaNO"_3 = 4.90 × 10^"-3" color(red)(cancel(color(black)("mol NaNO"_3))) × (6.022 × 10^23 "formula units NaNO"_3)/(1 color(red)(cancel(color(black)("mol NaNO"_3)))) = 2.95 ×10^21color(white)(l) "formula units NaNO"_3#