Question #b3a29
1 Answer
Feb 1, 2016
Paramagnetism is when a substance has an electron configuration with at least one unpaired electron.
A common example for a paramagnetic element is iron, atomic number
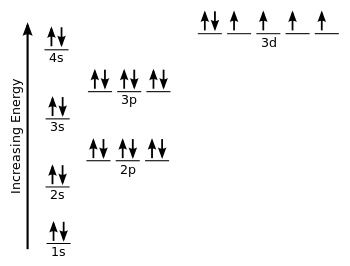
We should recognize that with
You only have
Therefore, iron is paramagnetic.
As a side note, a diamagnetic compound has no unpaired electrons at all. So, zinc is diamagnetic.

