How many sigma and pi bonds are present in a molecule of nicotine?
1 Answer
Here's what I got.
Explanation:
In order to be able to determine how many sigma bonds and how many pi bonds are present in a molecule of nicotine,
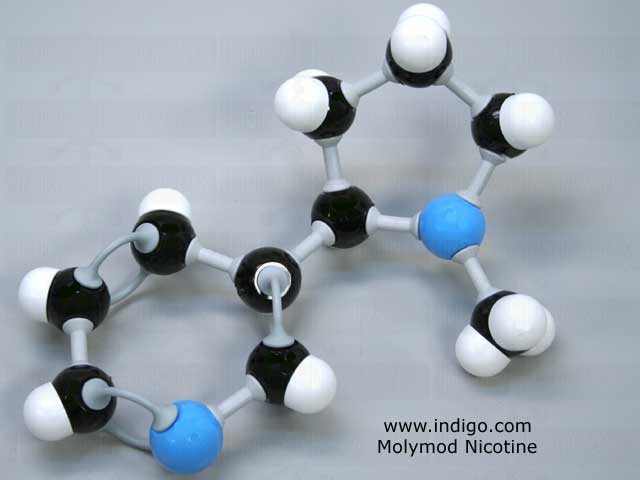
Here the nitrogen atoms are shown in blue, the carbon atoms in black, and the hydrogen atoms in white.
Now, your goal when looking for sigma and pi bonds is to first recognize how many single, double, and triple bonds are present in the molecule.
The key here is to keep in mind that
- every single bond is also a sigma bond
- every double bond contains a sigma bond and a pi bond
- every triple bond contains a sigma bond and two pi bonds
To make these bonds more visible, use the Lewis structure of the molecule
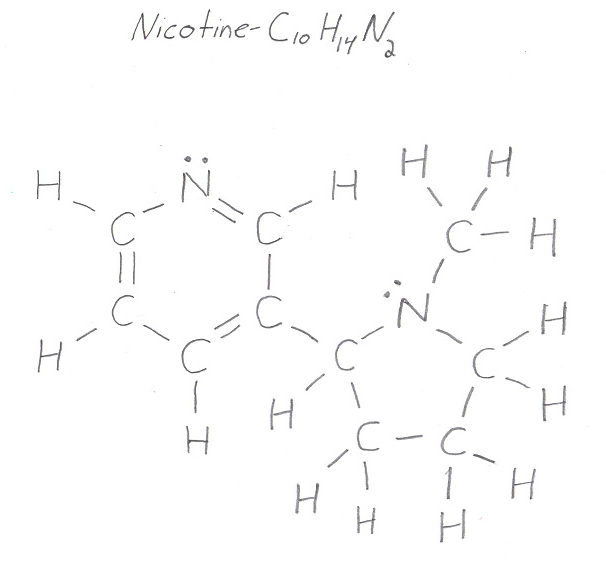
So, every single bond is also a sigma bond. Count the single bonds carefully to get
#sigma_"single" = 24 -># from single bonds exclusively
At the same time, every double bond contains one sigma and one pi bond. This means that you will also have
#sigma_"double" = 3 -># from double bonds exclusively
#pi_"bonds" = color(green)(3)#
Therefore, the total number of sigma bonds will be
#sigma_"total" = sigma_"single" + sigma_"double"#
#sigma_"total" = 24 + 3 = color(green)(27)#
The nicotine molecule wili thus contain
#{( 27color(white)(a)sigma"-bonds"), (3color(white)(a)pi"-bonds") :}#

