What will happen if I heat a solution of 1-bromo-1,2-dimethylcyclohexane in methanol?
1 Answer
The organic products are 1-methoxy-1,2-dimethylcyclohexane and 1,2-dimethylcyclohexene.
Explanation:
The structure of 1-bromo-1,2-dimethylcyclohexane is
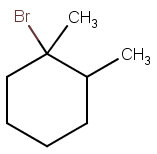
This is a 3° alkyl halide, so the mechanistic possibilities are
I predict a mixture of both, with the
The first step is loss of the bromine atom to form the tertiary carbocation.
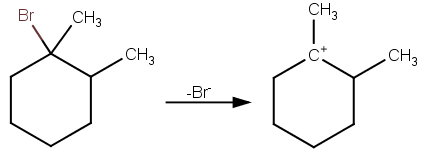
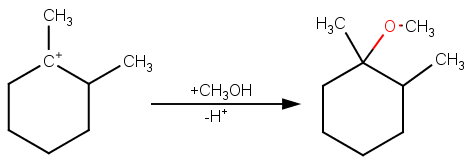
Then the methanol can attack the carbocation to form 1-methoxy-1,2-dimethylcyclohexane.
As an alternative, methanol could act as a base and remove an α-hydrogen in an
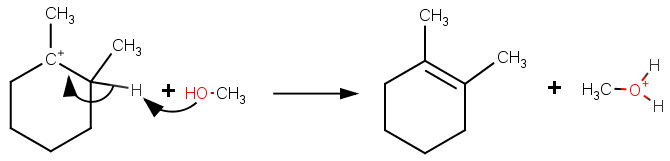
At higher temperatures, more molecules will have enough energy to get over the activation energy barrier for elimination, so the proportion of the cyclohexene product will increase.

