Question #9fa33
2 Answers
The product will be propane.
Explanation:
This reaction is the hydrogenation of an alkyne.
Hydrogenation is an addition reaction, in which one of the
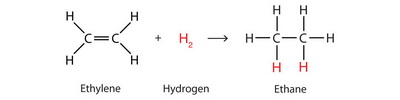
The addition requires a
With an alkyne such as propyne, we can think of the reaction as occurring in two steps.
(a) 1 mol of
(b) Another mole of
The overall reaction is
I think the questioner could have given more information, as the hydrogenation requires a catalyst.
From the question, with no specification of the catalyst, I can see two different reactions, both giving syn addition of
In general:
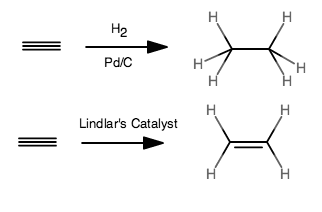
Lindlar's Catalyst is a "poisoned" variant on a palladium catalyst. It reduces alkynes to cis-alkenes, but doesn't reduce alkenes.


