Question #eaaa1
1 Answer
You would see (b) in the context of reactions involving gases. For instance:
#2"Al"(s) + 6"HCl"(aq) -> 2"AlCl"_3(aq) + 3"H"_2(g)#
GASES, LIQUIDS, AND SOLIDS CORRELATE WITH DECREASING MAGNITUDES OF ENTROPY
We know that gases have higher entropy than liquids, which have higher entropy than solids.
Here's a depiction of that relationship:
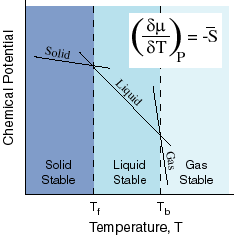
If we look at this diagram, we should notice that
Therefore, based on the steepness of the slopes, we should see that the magnitude of the entropy is much greater for a gas than for a liquid or solid.
This means that the above reaction has a net positive entropy change because it has more
#"mol"# s of gas on the products side.
DISTURBING THE EQUILIBRIUM
From applying the ideal gas law upon the hydrogen gas product, we should expect that if we increase the pressure at a constant volume on the above reaction, the reaction's temperature would increase.
Thus, since the x axis of the diagram has increasing temperature from left to right, we move to the right in the diagram, and the equilibrium would be disturbed in the direction of the products, since there are more
In response, Le Chatelier's principle says that the reaction would shift back over to the reactants side to re-establish equilibrium, i.e. shift towards the side with less
#\mathbf("mol")# s of gas.

