Question #6e583
1 Answer
Here's what I get.
Explanation:
The structure of glucose is
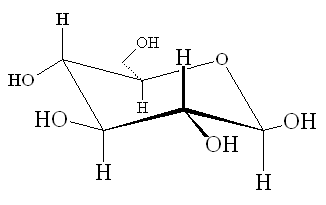
Electron domain geometries
Every carbon atom is directly attached to four other atoms (steric number = 4). Its electron domain geometry is therefore tetrahedral.
Every oxygen atom has two lone pairs and is directly attached to two other atoms (steric number = 4). Its electron domain geometry is therefore tetrahedral.
Molecular geometries
Every carbon atom is directly attached to four other atoms. Its molecular geometry is therefore tetrahedral.
Every oxygen atom has two lone pairs and two bonding pairs. Its molecular geometry is therefore bent.
Hybridization
Every carbon and oxygen atom has a steric number of 4. Their hybridization is therefore
Bond angles
All bond angles are approximately equal to the tetrahedral bond angle of 109.5°.

