Question #95d67
1 Answer
Feb 12, 2016
Those letters are used just by historical reasons.
Explanation:
The first time that atomic orbitals were observed, it was done by spectroscopists, which observed them as lines on the atomic spectra.
Here, there are some examples of atomic spectra for several elements:
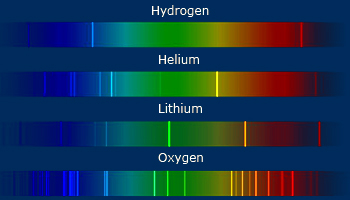
Source: www.wonderwhizkids.com
Each of the lines correspond to a transition to (or from) some of the blocks. They were called in that way according to how they were observed on alkali spectra:
#s# for#s# harp lines.#p# for#p# rincipal lines.#d# for#d# iffuse lines.#f# for#f# undamental lines.
After

