Question #05458
1 Answer
Let's find out!
Explanation:
Atoms are very, very tiny things, so in order to be able to determine the mass of a single atom you need to know the mas of a mole of atoms.
A mole is simply a very, very large collection of atoms, larger enough so that the mass of one mole of atoms can have sense for us.
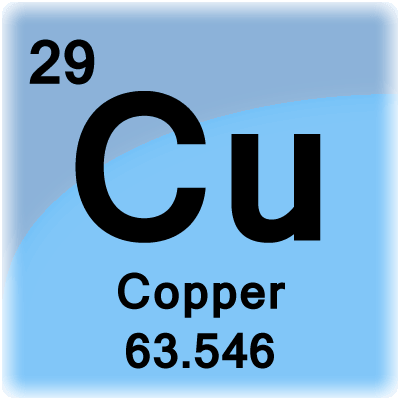
So, pick up a periodic table and look for copper,
That number tells you the mass of one mole of copper atoms, i.e. copper's molar mass. Copper has a molar mass of
Now, in order to have one mole of copper, you need to have exactly
So, if one mole of copper has a mass of
#1color(red)(cancel(color(black)("atom of Cu"))) * overbrace((1color(red)(cancel(color(black)("mole of Cu"))))/(6.022 * 10^(23)color(red)(cancel(color(black)("atoms of Cu")))))^(color(Purple)("Avogadro's number")) * overbrace("63.546 g"/(1color(red)(cancel(color(black)("mole of Cu")))))^(color(blue)("copper's molar mass")) = color(green)(1.055 * 10^(-22)"g")#
So the answer to your question is yes, one atom of copper has a mass of