One mole of sugar has a greater mass than one mole of water, how come?
1 Answer
Here's why that is the case.
Explanation:
First of all, it's always a good idea to start with what a mole actually means, that way you can be sure that you know what you're looking at here.
Atoms and molecules are very, very small, which of course implies that they have very, very small masses, much, much smaller than the scale we're used to in our daily lives.
In order to be able to convert this molecular scale to something that's more familiar to us, like grams, we needed to group a whole lot of these molecules together.
So, how many molecules do we need in order to have "a whole lot"? Avogadro's number,
So, in order to have a mole of sugar, for example, you need to have
So, why does one mole of sugar weight more than one mole of water?
Because an individual molecule of sugar weighs more than an individual molecule of water.
Sugar, which is the common name used for sucrose, has the chemical formula
#12# atoms of carbon#22# atoms of hydrogen#11# atoms of oxygen
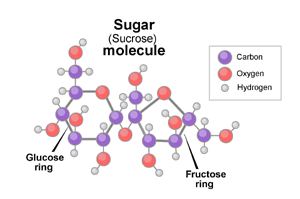
Water has the chemical formula
#2# atoms of hydrogen#1# atom of oxygen
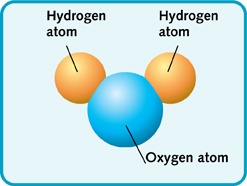
So one molecule of water will have a smaller mass than one molecule of sugar, since it contains fewer atoms.
This of course means that two molecules of water will have a smaller mass than two molecules of sugar.
The same goes for
This is why one mole of sugar has a bigger mass than one mole of water, because one molecule of sugar has a bigger mass than one molecule of water.

