Question #4afa9
1 Answer
... will decrease.
Explanation:
As you know, acetic acid,
This implies that acetic acid's dissociation in aqueous solution is actually a chemical equilibrium reaction.
#"CH"_3"COOH"_text((aq]) + "H"_2"O"_text((l]) rightleftharpoons "CH"_3"COO"_text((aq])^(-) + "H"_3"O"_text((aq])^(+)#
Now, an equilibrium reaction is governed by Le Chatelier's Principle, which states that a dynamic equilibrium will shift in such a way as to counteract any stress placed on the position of the equilibrium.
Sodium acetate,
#"CH"_3"COONa"_text((aq]) -> "CH"_3"COO"_text((aq])^(-) + "Na"_text((aq])^(+)#
So, when you're adding sodium acetate to a solution of acetic acid, you're essentially increasing the concentration of acetate anions.
This will affect the position of the equilibrium. As a result, a shift will take place aimed at reducing this stress.
More precisely, the equilibrium will shift to the left, favoring the reverse reaction. This shift to the left will consume acetate anions and produce molecular, unionized acetic acid.
This will also decrease the concentration of hydronium ions, and, as a result, increase the pH of the solution, i.e. make the solution less acidic.
Adding sodium acetate to a solution of acetic acid will create a buffer solution, i.e. a solution that contains a weak acid and its conjugate base in comparable amounts.
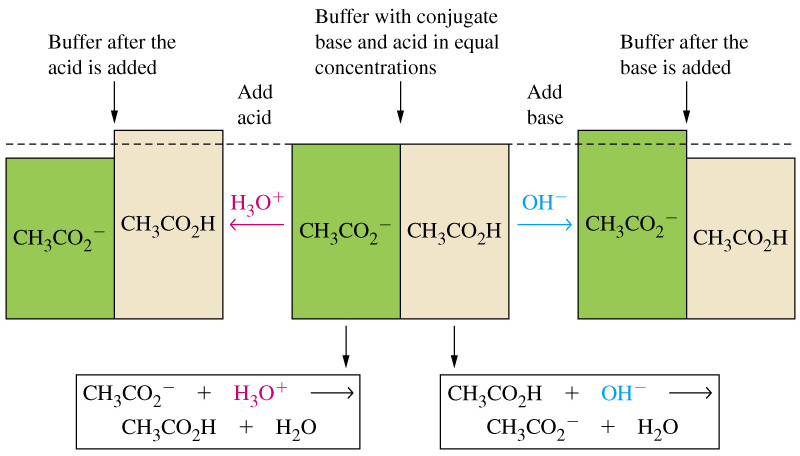
So, as a conclusion, the degree of dissociation of the acid will decrease, since the shift in the position of the equilibrium will result in a decrease in the concentration of acetate anions and hydronium cations.

