Let the chlorination reaction of Ethane (C_2H_6) with chlorine replaces n atoms from its molecule producing chloro ethane of formula C_2H_(6-n)Cl_n
Considering atomic masses as
C=12g/"mol"
H=1g/"mol"
Cl=35.5g/"mol"
We get the molar mass of chloroethane as
2xx12+(6-n)xx1+nxx35.5
=30+34.5n
And % Cl in the compound as calculated from its molecular formula C_2H_(6-n)Cl_n will be
=(35.5xxnxx100)/(30+34.5n)
Equating this with the given percentage we can write
(35.5xxnxx100)/(30+34.5n)=71.7
=> (35.5xxnxx100)/71.7=(30+34.5n)
=> 49.5n=(30+34.5n)
=> 49.5n-34.5n=30
=>n=30/15=2
(A) Hence the molecular formula of chloroethane is C_2H_4Cl_2
(B) The condensed structural formula and name of the isomers
CH_3CHCl_2->1,1-"dichloroethane"
CH_2ClCH_2Cl->1,2-"dichloroethane"
(C) Between the two isomers the major product will be
CH_3CHCl_2->"1,1-dichloroethane"
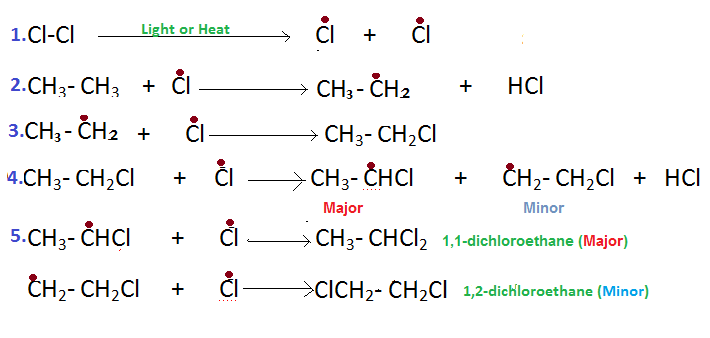
The reaction follows free radical mechanism.
(1) Cl* free radical is first produced by homolytic fission of Cl-Cl" "sigmabond under the influence of light or heat energy.
(2)This Cl* free radical extracts H atom from CH_3CH_3 molecule forming CH_3CH_2* free radical
(3) CH_3CH_2* free radical then combines with Cl* free radical to form CH_3CH_2Cl ,monochloroethane.
(4) Now the extraction of H"-atom" from CH_3CH_2Cl by Cl* again occurs with two possibilities forming CH_3CHCl* or *CH_2CH_2Cl
(5) The first one color(blue)(CH_3CHCl*) is more stable free radical as it has got more alpha"H in respect of free radical providing higher hyper conjugative effect. So the first one which is formed in higher concentration in the reaction mixture,will combine with Cl* free radical more and thus the major product will be
color(red)(CH_3CHCl_2->"1,1-dichloroethane")
 drawn
drawn
 drawn
drawn 
