Question #d45c9
1 Answer
Explanation:
Your strategy here will be to
- use glucose's molar mass to determine how many moles you have in that sample
- use Avogadro's number to determine how many molecules you have in that many moles
- use glucose's chemical formula to find how many atoms you get in that many molecules
So, glucose,
Use the molar mass of glucose as a conversion factor to determine how many moles you have in your
#843.211color(red)(cancel(color(black)("g"))) * overbrace(("1 mole C"_6"H"_12"O"_6)/(180.156color(red)(cancel(color(black)("g")))))^(color(blue)("molar mass of glucose")) = "4.68045 moles C"_6"H"_12"O"_6#
Now, Avogadro's number tells you how many atoms or molecules you get in one mole of a given substance. Since you know how many moles of glucose you have in your sample, you can use Avogadro's number as a conversion factor to help you go from moles to number of molecules
#4.68045color(red)(cancel(color(black)("moles"))) * overbrace((6.022 * 10^(23)"molec.")/(1color(red)(cancel(color(black)("mole")))))^(color(purple)("Avogadro's number")) = 2.81857 * 10^(24)"molec."#
To get the number of atoms you get in that many molecules of glucose, focus on figuring out how many atoms you get in one molecule first.
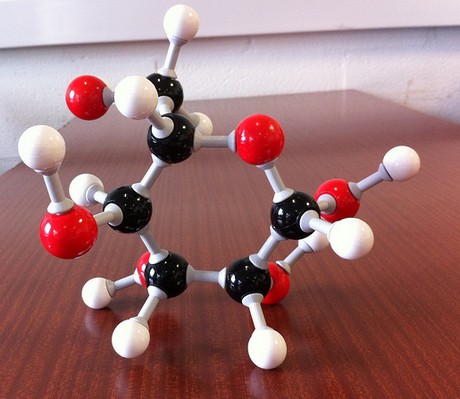
Each molecule of glucose is made up of
- six atoms of carbon,
#6 xx "C"# - twelve atoms of hydrogen,
#12 xx "H"# - six atoms of oxygen,
#6 xx "O"#
This means that one molecule of glucose will contain a total of
#6 + 12 + 6 = "24 atoms"#
The number of atoms present in your sample will thus be equal to
#2.81857 * 10^(24)color(red)(cancel(color(black)("molec."))) * "24 atoms"/(1color(red)(cancel(color(black)("molec.")))) = color(green)(|bar(ul(color(white)(a/a)6.76457 * 10^(25)"atoms"color(white)(a/a)|)))#
The answer is rounded to six sig figs, the number of sig figs you have for the mass of sucrose.

