Question #0e1e8
1 Answer
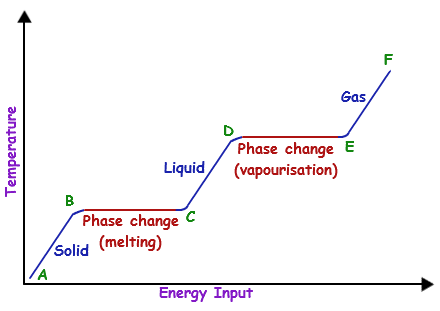
Temperature remains constant as heat (energy) is put in to break the intermolecular forces instead during a phase change.
Explanation:
When a sample changes phase (from Solid-> Liquid-> Gas) enough energy has to be put in to overcome the intermolecular forces between particles. For example, when boiling water it will stay at 100 degrees Celsius until energy has been put in to break the forces, and then you will have steam at 100 degrees that can now be heated even further. This graph makes a good illustration of what is happening: