How do you start from the line structure, then determine the hybridization in order to figure out the structure with explicit atoms, for 1,3,4-trimethyl-1-pentene? What is the molecular formula?
1 Answer
The idea is that organic chemists like drawing compounds to be convenient, so they "abbreviate" the structures like so:
"C"-="C" becomes three parallel lines (-= ), and each end of the lines has an implicit carbon."C"="C" becomes two parallel lines= ), and each end of the lines has an implicit carbon."C"-"C" becomes one line (- ), and each end of the lines has an implicit carbon.- All hydrogens on carbon atoms are implicitly there (unless the compound has only one or two carbons, in which case it would look nicer to write
"H"_n ). That means they are either not drawn or they are written as"H"_n . - All heteroatoms (non-carbon atoms) remain explicitly visible.
So, all you have to do is count atoms and tally up how many of each type you have. The challenge may come in:
- Converting from implicit to explicit sketches
- Identifying how many hydrogens are on a carbon based on its hybridization (
sp^3 -> three hydrogens per terminal carbon,sp^2 -> two hydrogens per non-terminal carbon,sp -> one hydrogen per terminal carbon). - Working out the approximate bond angles
The structure in an explicit sketch looks like this:
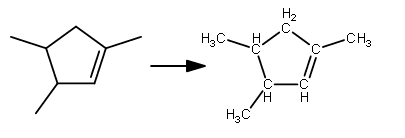
Now, we'd count the atoms to be:
-
"C" :8 3 of these carbons are primarysp^3 and thus have three hydrogens
2 of these carbons are tertiarysp^3 and thus have one hydrogen
1 of these carbons is a secondarysp^3 and thus has two hydrogens
1 of these carbons are secondarysp^2 and thus have one hydrogen
1 of these carbons are tertiarysp^2 and thus have no hydrogens -
"H" :14 9 of these are from the"CH"_3 's (bottom-left, upper-left, upper-right)
2 of these are from the"CH"R_1R_2 's (bottom-left, upper-left)
2 of these are from the"CH"_2 (top)
1 of these are from the-"CH"=("C"R_1R_2) (bottom right)
So the molecular formula is

