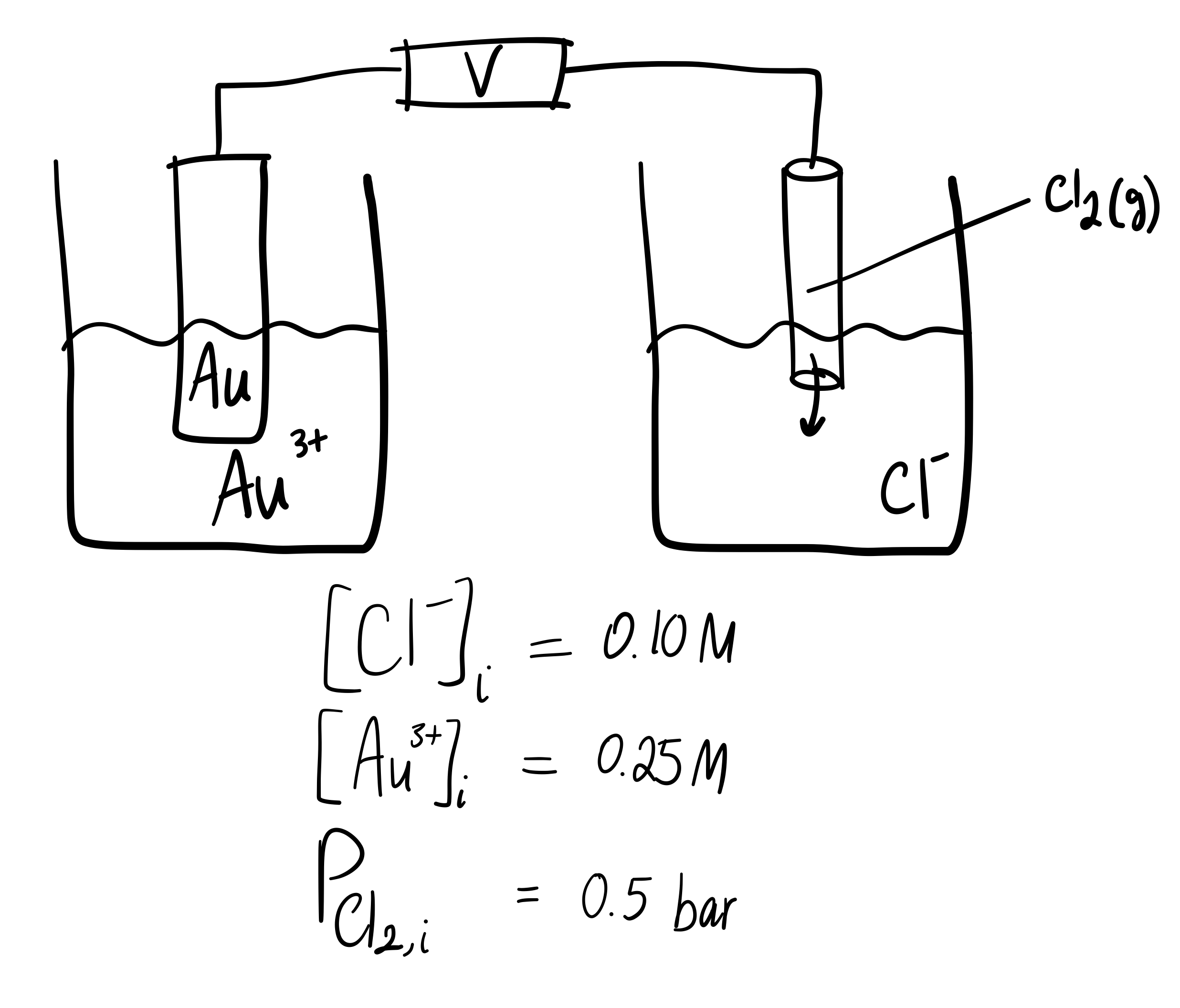
For the reaction of #"Au"^(3+)# with #"Cl"_2#, if #["Cl"^(-)]# and #["Au"^(3+)]# were initially #"0.10 M"# and #"0.25 M"# respectively, while #P_("Cl"_2) = "0.5 bar"# initially, what is #E_(cell)#?
Ecell=0.14V
Q=2x106
Ecell=0.07785V
Ecell=0.14V
Q=2x106
Ecell=0.07785V
1 Answer
Well, what I would do is draw out the problem to see what you got...
The Nernst equation is:
#E_(cell) = E_(cell)^@ - (RT)/(nF)lnQ#
#E_(cell)# is the cell potential at nonstandard#T# and#P# .#""^@# signifies#"1 bar"# and#25^@ "C"# .#R# and#T# are known from the ideal gas law, and are in#"J/mol"cdot"K"# and#"K"# , respectively.#n# is the mols of electrons transferred in the overall reaction.#F = "96485 C/mol e"^(-)# is the Faraday constant.#Q# is the reaction quotient.
From the looks of it, you are supposed to calculate
First, write out the half-reactions. You need to know how to look up reduction reactions in a standard reduction potential table, which is mandatory at any chemistry exam with electrochemistry questions.
#"Au"^(3+)(aq) + 3e^(-) -> "Au"(s)# ,#" "E_(red)^@ = "1.50 V"#
#"Cl"_2(g) + 2e^(-) -> 2"Cl"^(-)(aq)# ,#" "E_(red)^@ = "1.36 V"#
Since
#2("Au"^(3+)(aq) + cancel(3e^(-)) -> "Au"(s))# ,#" "E_(red)^@ = "1.50 V"#
#3(2"Cl"^(-)(aq) -> "Cl"_2(g) + cancel(2e^(-)))# ,#" "E_(o x)^@ = -"1.36 V"#
#"-------------------------------------------"#
#2"Au"^(3+)(aq) + 6"Cl"^(-)(aq) -> 3"Cl"_2(g) + 2"Au"(s)#
And its standard cell potential, i.e.
#color(blue)(ul(E_(cell)^@)) = E_(red)^@ + E_(o x)^@#
#= "1.50 V" + (-"1.36 V") = ul(color(blue)(+"0.14 V"))#
For this reaction, we have the reaction quotient, which is written just like
#Q = ((P_("Cl"_2(g))//P^@)^3)/((["Au"^(3+)(aq)]//c^@)^2(["Cl"^(-)]//c^@)^6)# where
#P^@ = "1 bar"# and#c^@ = "1 M"# such that#Q# is unitless. It must be, or else it cannot be operated on by#ln# .
We can immediately obtain the reaction quotient with the given data:
#color(blue)(ulQ) = ("0.5 bar"//"1 bar")^3/(("0.25 M"//"1 M")^2("0.10 M"//"1 M")^6)#
#= color(blue)(ul(2 xx 10^6))#
And that allows us to finally get the nonstandard cell potential. You didn't tell us the temperature, but I am guessing it is
#color(blue)(ul(E_(cell))) = "0.14 V" - (("8.314472 V"cdot"C/mol"cdot"K")("298.15 K"))/(("6 mol e"^(-)/("1 mol atom"))("96485 C/mol e"^(-)))ln(2 xx 10^6)#
#=# #"0.07787 V"#
#~~# #color(blue)(ul("0.07785 V"))#
And indeed it is at

