Question #ef968
1 Answer
Dec 26, 2016
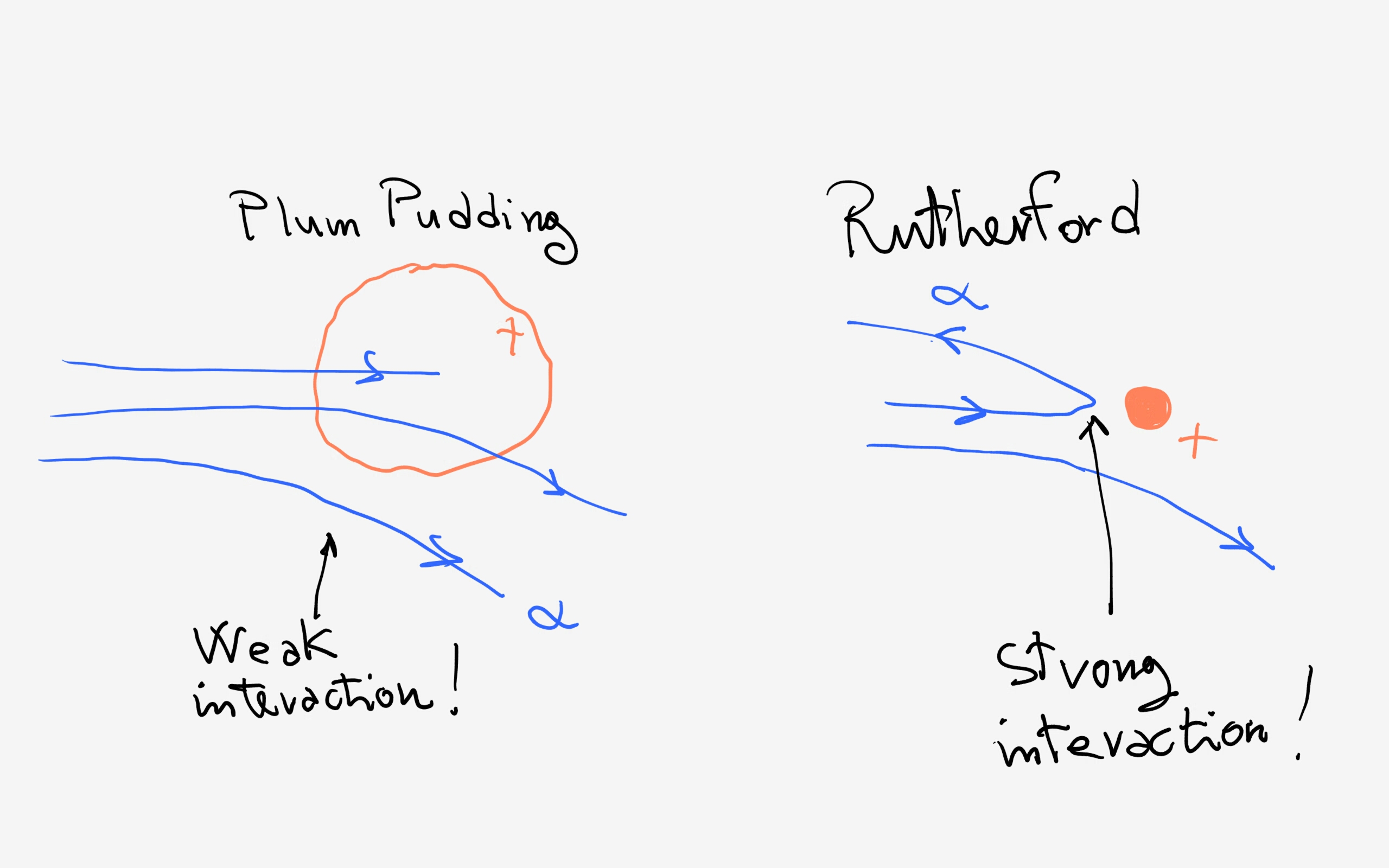
Because of the experimental results of Rutherford hinting at the presence of a concentrated positively charged entity inside the atom.
Explanation:
Rutherford bombarded gold atoms using, as bullets, positively charged alpha particles. The result was that not only some of the bullets were deviated (as expected) but some of them were ricocheting back!
This kind of behaviour could only be acounted for postulating the presence of a massive and concentrated positive charge and not a diffused "dough" of positive charge as postulated by Thomson.