Are all ionic compounds soluble in water?
1 Answer
Not all ionic compounds are easily soluble in water. Just because a compound is ionic doesn't mean it is automatically soluble in water.
A soluble compound defined by Sigma Aldrich is approximately
Examples of ionic compounds that are either borderline soluble, poorly soluble, or essentially not soluble in the least, are:
#"Ba"("OH")_2# (#"3.89 g/100 mL"# @#20^@ "C"# ;#K_(sp) ~~ 5.0xx10^(-3)# )#"CaSO"_4# (#~~# #"0.2 g/100 mL"# @#20^@ "C"# ;#K_(sp) ~~ 4.93xx10^(-5)# )#"AgCl"# (#~~# #"0.00052 g/100 mL"# @#20^@ "C"# ;#K_(sp) ~~ 1.77xx10^(-10)# )#"AgI"# (#~~# #"0.0000003 g/100 mL"# @#20^@ "C"# ;#K_(sp) ~~ 8.52xx10^(-17)# )#"PbS"# (...Let's just call it zero.#K_(sp) ~~ 9.04xx10^(-29)# )
And for each of the above, there contains a cation (
If an ionic compound does happen to be easily soluble in water, it must be that...
the ion-ion interactions between the cation and the anion are weaker than the ion-dipole interactions between each ion and water molecules.
When a solid dissolves successfully in water, it must break apart its ion-ion interactions, and form new ion-dipole interactions.
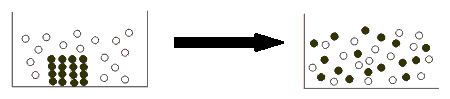
Basically, the idea is that the stronger interaction can more favorably form.
- If the interaction between the cation and the anion is weak, but the interaction between each ion and water is strong, the solid is favorably soluble.
- If the interaction between the cation and the anion is strong, but the interaction between each ion and water is weak, the solid is unfavorably soluble.

