Question #15e99
1 Answer
The IR photon.
Explanation:
The energy of a photon is directly proportional to its frequency as described by the Planck - Einstein relation
#color(blue)(|bar(ul(color(white)(a/a)E = h * nu color(white)(a/a)|)))#
Here
For two photons of frequency
#E_1 = h * nu_1" "# and#" "E_2 = h * nu_2#
If you were to divide these two equations, you would find
#E_1/E_2 = (color(red)(cancel(color(black)(h))) * nu_1)/(color(red)(cancel(color(black)(h))) * nu_2)#
Rearrange to get
#E_2 = nu_2/nu_1 * E_1#
If
If
Now, take a a look at the EM Spectrum.
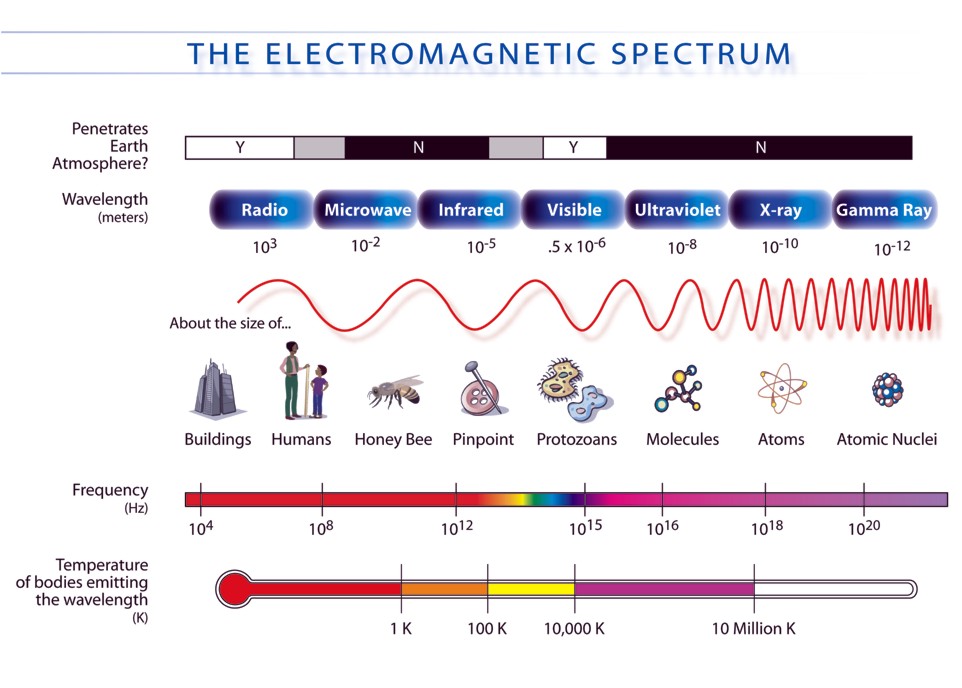
Notice that infrared frequency measures at about
Since a photon of IR radiation has a higher frequency than a photon of microwave radiation, it follows that it will also be more energetic.
In fact, you can say that you have
#E_"IR" = (10^12 color(red)(cancel(color(black)("Hz"))))/(10^9color(red)(cancel(color(black)("Hz")))) * E_"microwave"#
#E_ "IR"= 10^3 * E_"microwave"#
Therefore, a photon of frequency

