What is the geometry of the interhalogen, #IF_7#?
1 Answer
Jun 28, 2017
Well, we could predict its structure by VESPER........
Explanation:
We have
From the wiki link......
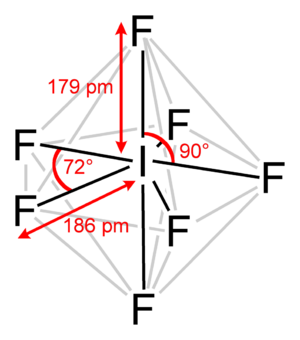
This is not something I would like to handle.
And how would you prove the structure? This is not a straightforward proposition: x-ray crystallography; solution NMR by

