Question #b1cb9
1 Answer
Aug 22, 2016
Do you mean calcium oxide and water, by any chance?
Explanation:
I don't think that calcium oxide,
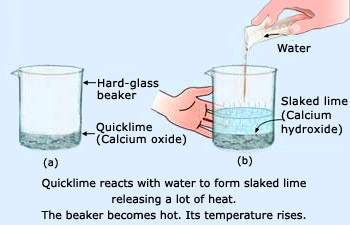
Calcium oxide, also called quicklime, will react with water to form calcium hydroxide,
#"CaO"_ ((s)) + "H"_ 2"O"_ ((l)) -> "Ca"("OH")_ (2(s))#
Calcium hydroxide is relatively soluble in water, but the amount that does dissolve dissociates completely to produce a basic solution of
It's worth mentioning that this reaction is quite exothermic, meaning that heat is being given off as calcium hydroxide is produced by the reaction.