Question #ed86f
1 Answer
I found a similar question in:
https://socratic.org/questions/why-was-j-j-thomson-wrong
...answered by far more competent expert than me!
Explanation:
Thomson (although easy to misspell with an extra "p") considered the atom as a dense positive spherical structure dotted with small point like negative structures.
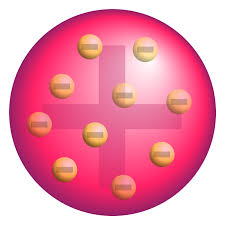 www.boundless.com
www.boundless.com
It was called the "Plum-Pudding Model" for this kind of particular conformation.

Remember that Thomson had recently discovered through a very genial experiment the "existence" and characteristics (mass/charge ratio) of the electron so he was in the perfect spot to suggest a model.
His model was not very good when dealing with positive constituent because it imagined it dispersed and not concentrated in a central structure, the nucleus, as it was subsequently demonstrated by the incredible experiment of Rutherford.

