Question #98443
1 Answer
Here's what I got.
Explanation:
Nickel(II) chloride,
The idea here is that both reactants are soluble ionic compounds, as shown by the solubility rules listed below
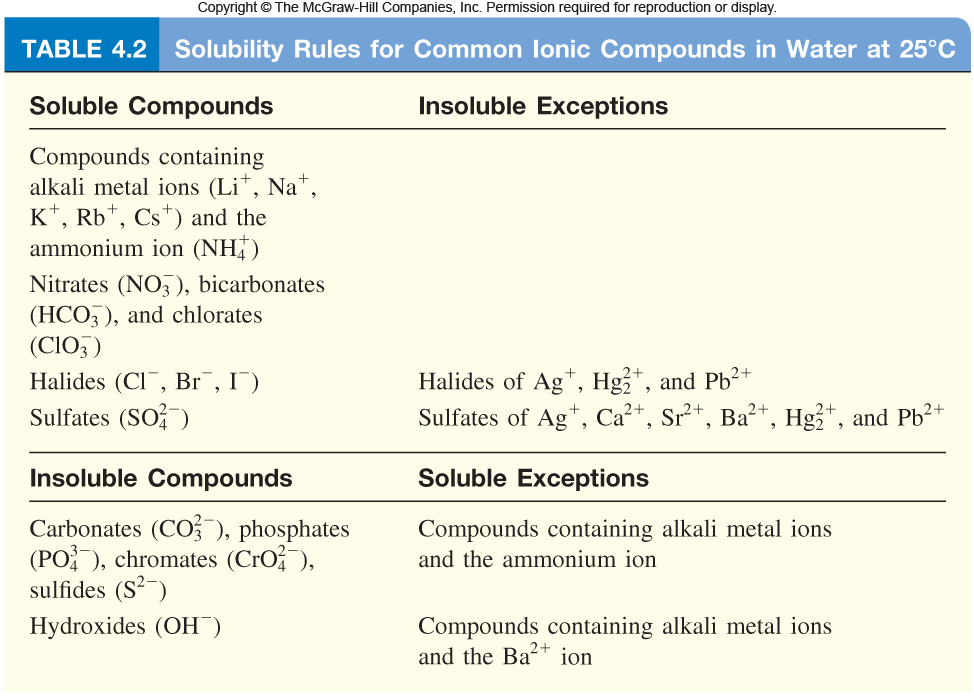
Nickel sulfide is an insoluble solid that precipitates out of solution, while ammonium chloride is a soluble ionic compound that will exist as ions in the solution, i.e. the reaction produces aqueous ammonium chloride.
You will thus have
#"NiCl"_ (2(aq)) + ("NH"_ 4)_ 2"S"_ ((aq)) -> "NiS"_ ((s)) darr + 2"NH"_ 4"Cl"_ ((aq))#
To get the net ionic equation, start by writing out the complete ionic equation
#"Ni"_ ((aq))^(2+) + 2"Cl"_ ((aq))^(-) + 2"NH" _ (4(aq))^(+) + "S"_ ((aq))^(2-) -> "NiS"_ ((s)) darr + 2"NH"_ (4(aq))^(+) + 2"Cl"_ ((aq))^(-)#
Eliminate the spectator ions, i.e. the ions that are present on both sides of the equation
#"Ni"_ ((aq))^(2+) + color(red)(cancel(color(black)(2"Cl"_ ((aq))^(-)))) + color(red)(cancel(color(black)(2"NH"_ (4(aq))^(+)))) + "S"_ ((aq))^(2-) -> "NiS"_ ((s)) darr + color(red)(cancel(color(black)(2"NH"_ (4(aq))^(+)))) + color(red)(cancel(color(black)(2"Cl"_ ((aq))^(-))))#
to get
#"Ni"_ ((aq))^(2+) + "S"_ ((aq))^(2-) -> "NiS"_ ((s)) darr#

