What is the standard cell potential for the reaction of silver cation with aluminum metal?
#A)# #"2.47 V"#
#B)# #"0.87 V"#
#C)# #"0.73 V"#
#D)# #"1.67 V"#
1 Answer
I get A.
As a short review,
The most reasonable reaction to occur is the one in which
From the activity series below:
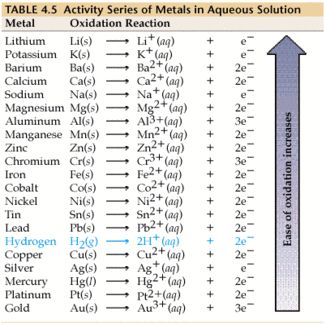
we should notice that elements higher up are more easily oxidized. In order for the overall reaction to be spontaneous, it must be that aluminum is oxidized, since it is more easily oxidized.
Or, you may have been given a series of reduction half-reactions instead, in which case, if
Therefore, we should flip the second reaction and get:
#3(Ag^(+)(aq) + e^(-) -> Ag(s))# ,#E_"red."^@ = +"0.80 V"#
#-(Al^(3+)(aq) + 3e^(-) -> Al(s))# ,#-E_"red."^@ = -(-"1.67 V")#
#"-------------------------------------------------"#
#3Ag^(+)(aq) + Al(s) -> 3Ag(s) + Al^(3+)(aq)# ,#E_"cell"^@ = ???#
Although
So,
If we did NOT scale the first reduction half-reaction by
#color(blue)(E_"cell"^@) = E_"cathode"^@ + E_"anode"^@#
#= E_"red."^@ + E_"ox."^@#
#= E_("red.","Ag")^@ - E_("red.","Al")^@#
#= "0.80 V" - (-"1.67 V") = color(blue)("2.47 V")#
which is A.
If we DID scale the first reduction half-reaction by
#cancel(color(red)(E_"cell"^@) = E_"red."^@ + E_"ox."^@)#
#= cancel(color(red)(3)E_("red.","Ag")^@ - E_("red.","Al")^@)#
#= cancel(3("0.80 V") - (-"1.67 V") = color(red)("4.07 V"))# ,
which is not one of the answer choices.

