Question #295d0
1 Answer
Explanation:
To do this type of question you need to construct a Hess Cycle.
Hess' Law states that the overall enthalpy change of a chemical reaction is independent of the route taken.
All the substances are made from the same elements so the elements can go on the bottom.
Then construct the cycle:
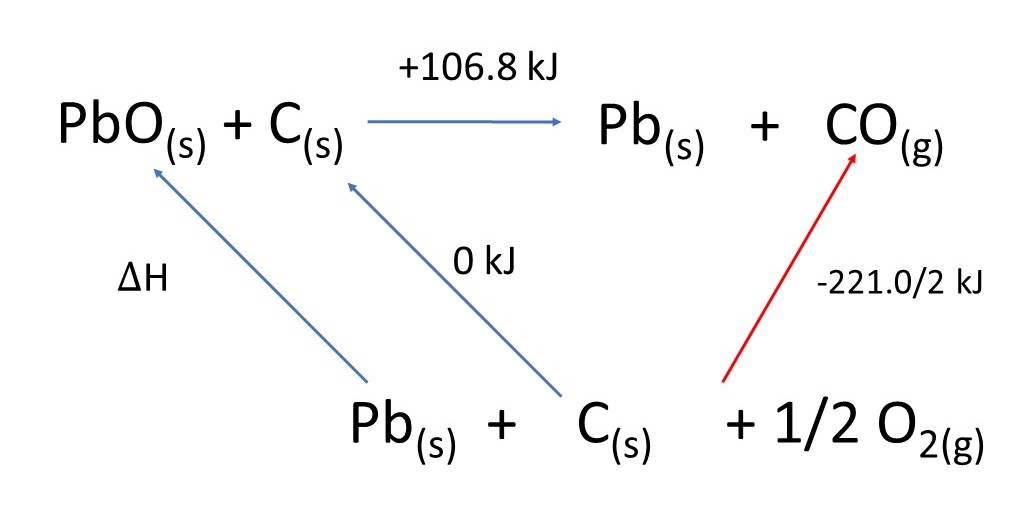
Note that I have divided the enthalpy of formation of
Also note that the enthalpy formation of the element carbon is zero.
Hess' Law tells us that the enthalpy change of the
This is because the arrows start and finish in the same place.
Applying the law
This refers to
The
So 1 mole of Pb weighs 207.2 g.
From the equation we can say:
The minus sign tells us that

