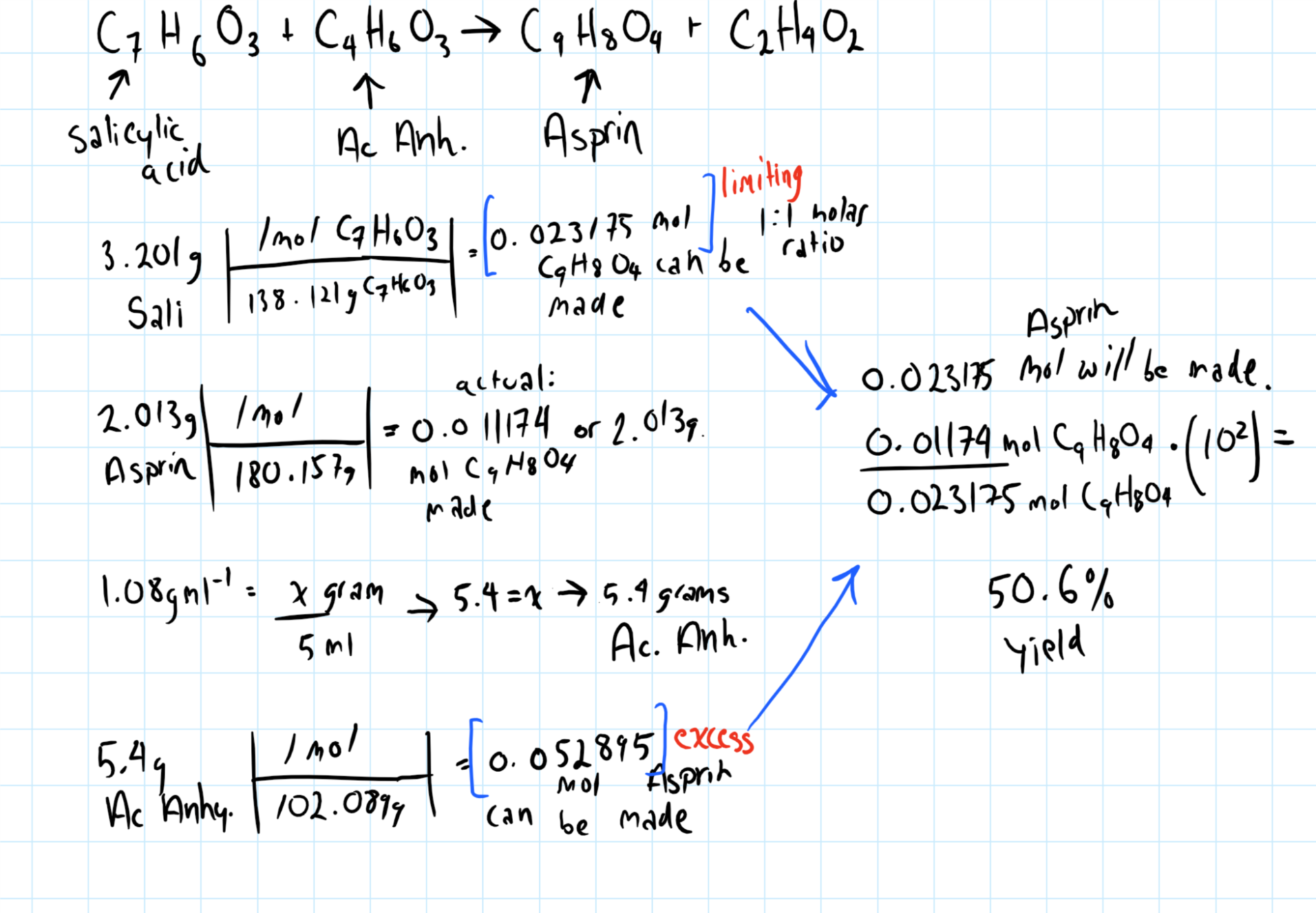Question #39bd1
1 Answer
Dec 5, 2016
50.6%
Explanation:
Start with a balanced chemical reaction, always. You need the molar ratios to determine percent yield.
In this case you could remember that they are 1:1, but I'm going to suggest you always find the balanced reaction, because in many cases the molar ratio will not be 1:1.
Remember to find the molar ratios and that the formula for percent yield is the actual/theoretical yield multiplied by 100.
Consider the graphic bleow: