Question #4310f
1 Answer
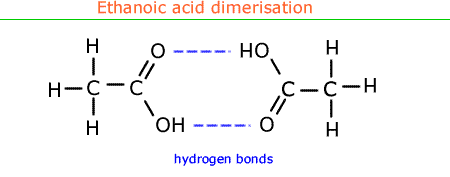
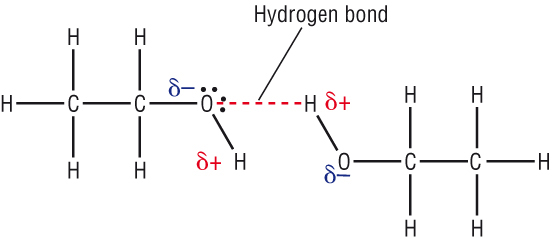
In ethanoic acid dimerisation occurs forming two H-bonds between two molecules making effective molar mass double as shown above.
In ethanol H-bond is also formed as shown above but dimerisation does not occur. So molecular association is less than that of ethanoic acid
In ethanal no
Here the molecules are associated with less strong dipole-dipole attraction.
So their boiling points are in the following order


