Question #77452
1 Answer
The charge for hydrogen is 1+, and sulfate is 2-.
Explanation:
Sulfuric acid,
What I personally do, is reverse the subscripts:
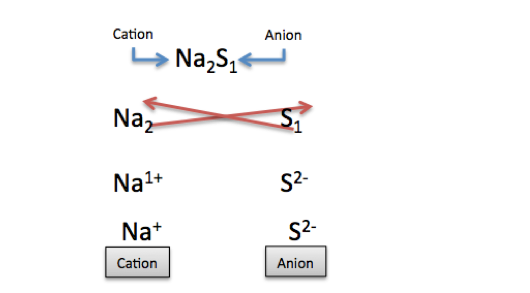
What's happening in the image is sodium's subscript changes into a superscript, transferring to the other atom, thus sulfur's charge is 2. We know sodium is a cation and sulfur is an anion, so the electrical charge is -. Therefore, the charge for sulfur is 2-. We do the same for sodium.
In
We do the same as
The charge for hydrogen is 1+, and sulfate is 2-.
Hope this helps :)

